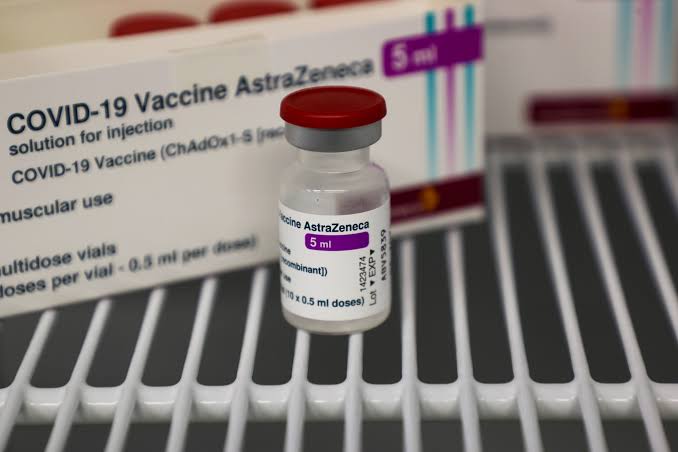
Hanoi, May 10, 2024, The Europe Today: Vietnam’s pursuit of COVID-19 vaccination efforts faces a setback as the Drug Administration of Vietnam, operating under the Ministry of Health, announced the depletion of AstraZeneca vaccines due to the expiration of their shelf life. The absence of new imports exacerbates the situation, leaving the country reliant on remaining stocks of other vaccine brands for pandemic control measures.
With AstraZeneca’s COVID-19 vaccine no longer available, Vietnam grapples with the challenge of ensuring continued immunization efforts. The vaccine’s global withdrawal, attributed to dwindling demand, underscores broader shifts in vaccination strategies worldwide.
Once a cornerstone of Việt Nam’s vaccination campaign, AstraZeneca’s COVID-19 vaccine garnered recognition from the World Health Organization (WHO), earning a place in the Emergency Use Listing amid the pandemic. Vietnam procured over 30 million doses of the vaccine from AstraZeneca, complementing donations from other sources.
The cessation of AstraZeneca vaccine administration in Vietnam predates July 2023, as confirmed by the national drug administration. Despite recent acknowledgments from AstraZeneca regarding rare side effects associated with the vaccine, health experts reassure the public, citing the low incidence rate of adverse reactions.
As Vietnam navigates the evolving landscape of COVID-19 vaccination, attention shifts towards optimizing existing vaccine reserves and diversifying procurement channels to sustain immunization efforts in the face of emerging challenges and uncertainties.