New York, December 08, 2023, The Europe Today: The United States Food and Drug Administration (FDA) Friday approved a landmark gene-editing treatment for sickle cell disease.
The US drug regulator “approved two milestone treatments, Casgevy and Lyfgenia, representing the first cell-based gene therapies for the treatment of sickle cell disease,” it said in a statement.
As DW reported last month, Exa-cel, using the brand name Casgevz, is based on CRISPR, a Nobel Prize-winning gene editing tool, used to snip patients’ DNA.
Casgevy was also approved by Britain’s Medicines and Healthcare Products Regulatory Agency last month.
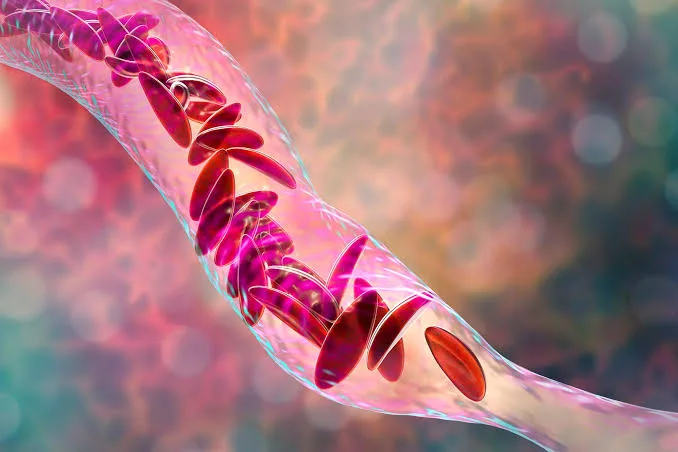
The new medication, Casgevy, targets the problematic gene in a patient’s bone marrow stem cells. It results in the production of properly functioning hemoglobin, the protein in red blood cells that carries oxygen, in the body.
Patients first receive a course of chemotherapy before doctors take stem cells from the patient’s bone marrow, and then those cells are treated with the medication in a lab, which, using the CRISPR gene editing tool, essentially cuts out the sections of DNA that cause the sickle shape.
The treated stem cells are reintroduced into the body.
Gene therapy treatments can cost millions of dollars and experts have raised concerns that they could remain out of reach for the people who would benefit most.
The other treatment approved by the FDA, Lyfgenia, utilizes a harmless virus to insert a gene into patients’ stem cells.
Before Casgevy and Lyfgenia were approved treatments included medications and blood transfusions.
The only permanent solution was a bone marrow transplant, which must come from a closely matched donor without the disease and brings a risk of rejection.