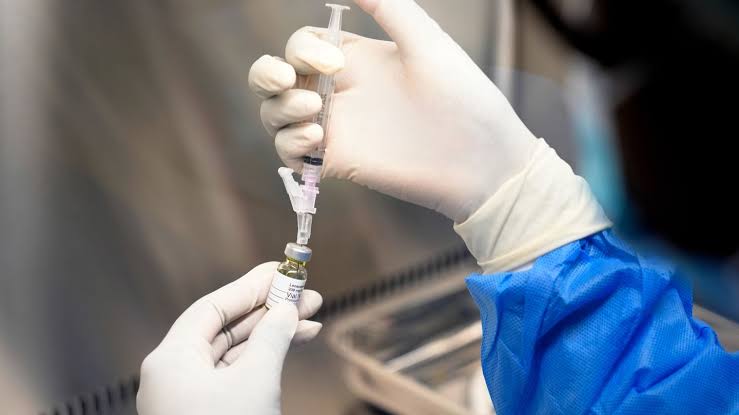
Brussels, August 31, 2025 — The Europe Today: The European Commission has approved a new preventive HIV treatment: a biannual injection developed by American pharmaceutical company Gilead.
Unlike current prevention methods, which involve daily pills or bimonthly injections, the new treatment can be administered by general practitioners during a regular doctor’s visit, offering a simpler and more accessible option for patients.
The drug, marketed as Yeztugo in the United States since June, will soon be available in Europe under the brand name Yeytuo after receiving clearance from the EU Committee for Medicinal Products for Human Use (CHMP).
According to Gilead’s clinical trials, the injection reduces the risk of HIV infection in adults and adolescents by more than 99.9%, making it one of the most effective preventive tools developed to date. Experts have hailed the approval as a potential breakthrough in the global fight against HIV.
However, concerns remain about accessibility and cost. In the United States, Yeztugo is priced at more than $28,000 (approximately €24,000) per year, raising questions about affordability in Europe.
Each year, around 25,000 new HIV cases are reported in Europe. Professor Jean-Michel Molina, an infectious diseases specialist, noted that these figures “clearly demonstrate that current prevention methods are not working for everyone who needs them.”